What do Preeclampsia, Heart Disease, and Adverse Vaccine Reactions have in Common? Cholesterol Sulfate Deficiency!
Those of you who have read my previous blog posts know by now that I am a big fan of cholesterol sulfate. The more I study, the more I come to realize that, more than any other factor, cholesterol sulfate deficiency is behind most of the conditions/diseases we face today. Perhaps the single largest factor contributing to cholesterol sulfate deficiency is inadequate sun exposure to the skin, combined with the interference effect of the chemicals in sunscreen.
Recently, I have been trying to learn everything I can about preeclampsia, in part because I think it is the most compelling example I have of cholesterol sulfate deficiency. Preeclampsia, also known as toxemia, is a serious condition now affecting 6-8% of pregnancies in the United States. Furthermore, a preeclampsic pregnancy is a significant risk factor (P=-0.0001) for future autism in the fetus [14]. Despite much study, the underlying cause of preeclampsia remains elusive. It begins innocently enough as elevated blood pressure and excess protein in the urine (proteinurea), but can quickly cascade into a remarkably divese set of alarming symptoms, sometimes terminating in the death of either the mother or the fetus or both.
Preeclampsia typically develops in the last trimester of pregnancy, the same period during which the placenta normally stocks up on cholesterol sulfate [11], as I have discussed previously. Is this a mere coincidence? I don’t think so! Proteinurea comes about because the glomeruli of the kidney allow large blood proteins like albumin and globulin to sneak past the sieve of their filter. An enlightening article published in the year 2000 [18] showed that such defective filtering in the kidneys is a consequence of both a decreased production of heparan sulfate and an increased breakdown of heparan sulfate by both reactive oxygen species (ROS) and active enzymes like heparanase. In my interpretation, the glomeruli are sacrificing their sulfate supply for the sake of the fetus, and this leads to a pathology in their function. In the extreme case, it can result in kidney failure, and this is one of the conditions that leads to death of the mother in severe preeclampsia.
Common Grounds in Preeclampsia and Cardiovascular Disease There are remarkable similarities between preeclampsia and cardiovascular disease. First of all, women who develop preeclampsia are at a greater risk to cardiovascular disease later in life [20]. Secondly, the serum markers for preeclampsia and cardiovascular disease are identical – high levels of small dense LDL particles and reduced serum HDL [21], elevated blood pressure, and high serum homocysteine [13]. Finally, preeclampsia is associated with a vascular disease in the arteries of the placenta that eerily resembles the defects in the arteries supplying the heart associated with cardiovascular disease – thickening of the artery wall, smooth muscle cell proliferation, and the appearance of fatty deposits in foam cells derived from macrophages [7,10].
Since I know that the placenta needs to accumulate a huge supply of cholesterol sulfate during the third trimester, I hypothesize that the purpose of the activities in the artery wall of the diseased placenta might be to produce cholesterol sulfate. This is further supported by two of the most effective treatments for preeclampsia: heparin and magnesium sulfate injections. While it is generally believed that it’s the magnesium in magnesium sulfate that’s important, I think it could well be that the sulfate is as well. And heparin, as I’ve said before, is the most highly sulfated molecule known to biology. Interestingly, heparin is also used as a treatment for infertile women who have become pregnant through in vitro methods.
Since cardiovascular disease has been much more thoroughly studied, I turn to the cardiovascular disease literature to answer my question – I seek evidence that cholesterol sulfate is being produced in the atheroma, and, by analogy, I suggest that this is also the reason for the disturbances in the artery wall of the placenta in preeclampsia. By deduction, I propose that the need to synthesize cholesterol sulfate is the underlying cause of both conditions.
The most significant evidence comes from the known association of both conditions with elevated serum homocysteine. Homocysteine is a sulfur-containing amino acid, and, when its level is elevated in the blood, much of it gets converted to homocysteine thiolactone, which then enters the artery wall in the atheroma and attaches itself to the matrix proteins [15]. A chemical reaction requiring superoxide (ROS) as a catalyst can oxidize the sulfur atom in homocysteine to produce sulfate. This reaction is catalyzed by vitamin C, which has been shown to reduce risk to preeclampsia when used as a supplement [5] [because it would enhance the supply of sulfate and hence solve the core problem]. Reaction with ATP then produces a molecule awkwardly called 3’-phosphoadenosine 5’-phosphosulfate (PAPS) [24], which is basically an energized sulfate molecule.
Platelets, also residing in the plaque, can synthesize cholesterol sulfate from cholesterol and PAPS. Experiments conducted in vitro have shown that platelets increase their synthesis rate of cholesterol sulfate 300 fold when they are provided with PAPS [24]. Red blood cells play an important role as well, because they provide ATP to the artery wall [23]. Cells are stimulated to produce ROS when they are exposed to externally supplied ATP, and I think this is because the ATP provides the oportunity to produce PAPS, but the ROS are needed first in order to derive sulfate from homocysteine thiolactone.
Platelets are also choosey about who can deliver the cholesterol to combine with the sulfate. In fact, they will only use cholesterol provided by HDL-A1 [24]. Reduced serum HDL, as well as reduced cholesterol content in serum HDL, are strong risk factors for cardiovascular disease. I therefore think that HDL is the bottleneck in the production of cholesterol sulfate. The macrophages in the plaque perform a vital function in extracting cholesterol from damaged small dense LDL particles, buffering it internally within fatty deposits, and later exporting it into HDL-A1, whenever the opportunity presents itself [9].
Since low cholesterol in HDL is a significant risk factor for atherosclerosis, how is HDL supposed to be supplied with cholesterol normally? Fibroblasts in the skin are an important supplier, mediated by ATP-binding Cassette A1 (ABCA1), and defective ABCA1 is associated with a substantially increased risk to atherosclerosis, along with decreased efflux of cholesterol from these peripheral cells [2]. I believe that cholesterol sulfate may be an intermediary that allows cholesterol to easily migrate from the cell wall of a fibroblast to the HDL monolayer. It has been demonstrated that cholesterol sulfate moves much more freely from one lipid membrane to another than does cholesterol, largely due to its increased solubility in water (i.e., blood) [19]. Fibroblasts are among the cell types that contain eNOS, and I have argued previously that eNOS can synthesize sulfate in the presence of sunlight. So I firmly believe that sunlight exposure to the skin would help fibroblasts fill the coffers of HDL containers with cholesterol. It follows that insufficient sunlight exposure would therefore lead to depleted cholesterol supplies in HDL, and therefore increased risk to cardiovascular disease and to preeclampsia.
Nitric Oxide, Cobalamin Destruction and Pernicious AnemiaIn a moment, I will show you some results I obtained by investigating adverse reaction reports from the VAERS (Vaccine Adverse Event Reporting System) database. In browsing the data, I was struck by the number of symptoms that were common in extreme vaccine reactions that are also associated with pernicious anemia. These include diarrhea, constipation, fatigue, light-headedness, appetite loss, pale skin, shortness of breath, swolen tongue, depression, loss of balance, and numbness/tingling. Pernicious anemia is due to a severe cobalamin (vitamin B12) deficiency, usually due to impaired uptake from the gut. However, cobalamin can be stored for a long time in the liver (up to a year), and so impaired uptake takes considerable time before it manifests as severe disease. In adverse vaccine reactions the symptoms come on very quickly, so it is likely to be due to a destruction of the cobalamin already present in the blood rather than to an impaired uptake.
Therefore, I sought papers that might explain how cobalamin could be destroyed. I came across several papers that discussed interactions between various oxides of nitrogen and cobalamin [8, 3, 16]. It seems that nitrous oxide (N2O), nitric oxide (NO) and peroxynitrite (ONOO−) all react with cobalamin to form various oxidized or nitrosylated derivatives. Since nitric oxide is the gas that’s produced by eNOS in the endothelium, it is the most likely candidate for our purposes, although the other two molecules will also be present as derivatives when NO is in high concentration in the blood.
Profuse nitric oxide synthesis is an expected reaction in the vasculature to exposure to endotoxins from pathogens, which are the “active ingredients” in vaccines. Furthermore, aluminum, added as an adjuvant, has also been shown to stimulate nitric oxide synthesis in the artery wall, likely due to its imitation of calcium, a well-established inducer of the reaction. A very enlightening paper [4] showed that anaphylactic shock (equivalent to an extreme adverse reaction to vaccines) can be induced in mice by exposing them to endotoxin and aluminum hydroxide. The paper elegantly demonstrated that it was eNOS (endothelial NOS, the constitutive form), not iNOS (the inducible form that macrophages produce to fight infection) which produced the profuse amounts of nitric oxide that led to anaphylactic shock. The anaphylactic shock reflects a sudden dramatic drop in blood pressure, brought on by the excess nitric oxide – a well known vasodilator. The journal editors, in an article introducing the above article [12], suggested that “agents that inhibit NOS or that scavenge NO might prove useful in treating life-threatening anaphylactic shock.” I maintain that one such agent is cobalamin!
A direct quote from a 1996 paper on nitric oxide and cobalamin says it better than I could ( [3], p. 1863): “Based on spectroscopy of urine samples, they believed that nitrosylcobalamin was formed in vivo in the mice overproducing NO as a result of endotoxin injection [34], and that the nitrosylcobalamin was being eliminated in the urine. Thus, it appears the H2O-Cbl [water-cobalamin complex] may bind NO and quench its effects both in vitro and in vivo.”
Thus cobalamin performs a useful role as a scavenger of excess nitric oxide, but then the reaction product is eliminated through the kidneys, and this will deplete the supply of cobalamin to the body. For someone who is deficient in cobalamin, this could then lead directly to a physiological state that’s indistinguishable from pernicious anemia.
Studies on the VAERS DatabaseI have done a number of studies of different subsets of the VAERS (Vaccine Adverse Event Recording System) database, which very nicely reveal a common thread among severe vaccine adverse reactions, autism, pernicious anemia and preeclampsia. My approach was to produce the following two datasets:
1. “
Autism set:” A subset of all cases where the word “autism” or the word “autistic” show up.
2. “
Anemia set:” A subset of all cases where any of the following symptoms show up: diarrhea, fatigue, light-headedness, loss of appetite, shortness of breath, swollen tongue, and numbness. These are all known symptoms of pernicious anemia.
The “autism” set consisted of 1323 events, and the “anemia” set was much larger, with over 50K events. I could then create another two sets, which I call “not-autism” and “not-anemia.” These were randomly drawn from the remainder of the (over 340,000) events, so as to maintain the same age distribution as the corresponding available sets for autism and anemia.
Now, what can be done is to count word frequencies for each of these pairs: Autism/Not-autism and Anemia/Not-anemia. Words that show up statistically significantly more frequently in the autism set or in the anemia set become words of interest, representing, collectively, other characteristics associated with the people who experienced these adverse reactions.
First of all, it’s reassuring that the word “anemia” was highly associated with the Anemia set (p=0.00074). Since we didn’t select on “anemia” itself, what this tells us is that the symptoms of anemia associate with anemia itself, as would be expected. Furthermore, the Anemia set is also an excellent predictor of autism (p=0.00066). What this tells us is either that autistic children are more likely to develop this anemia-like reaction to vaccines than other children, or that this group of symptoms is more likely to lead to autism. We can’t identify the cause-and-effect relationship, only the correlation, but it is very strong.
Another very interesting result we observed is that all of the symptoms that showed up with increased frequency in the Autism set were also highly overrepresented in the Anemia set. These include “anxiety,” “eczema,” “asthma,” “premature,” “pneumonia.” and “infection.” Thus, the Anemia set is a much larger set than the autism set which however captures the same set of conditions as the autism set, suggesting that the autism group of children are a small subset of a profile that is characterized by this anemia-like reaction to vaccines. The advantage of the Anemia set over the Autism set is that it is much larger, and therefore has a lot more statistical power in uncovering other related features.
So now, the interesting part comes when we look at additional symptoms/conditions that are over-represented in the Anemia set, beyond those present in the Autism set. I show these in Tables 1 and 2. Table 1 shows all the symptoms that are both highly overrepresented in the Anemia set and characteristic symptoms of preeclampsia. These include highly specific things like “blurry vision,” “facial swelling,” and “sensitivity to light,” as well as “pulmonary disease.” Table 2 shows additional symptoms that are over-represented in this set, which are cause for alarm, words like “seizure,” “death,” “paralysis,” and “heart failure.” These represent the final stage in a cascade reaction in the blood, when it is unable to right itself in time, following exposure to the toxins in the vaccine. They are also the extreme symptoms that show up in final stages of severe preeclampsia.
| Reaction | Count Anemia | Count Control | P-value |
Nausea | 8817 | 3088 | 4.2E-14 |
Headache | 4495 | 1839 | 9.5E-10 |
Abdominal Pain | 945 | 146 | 8.3E-7 |
Anxiety | 1720 | 728 | 6.7E-6 |
Pulmonary Disease | 453 | 113 | 0.00016 |
Vision Blurred | 420 | 129 | 0.00042 |
Visual Impairment | 258 | 54 | 0.00069 |
Facial Swelling | 288 | 162 | 0.015 |
Eye Irritation | 119 | 50 | 0.022 |
Sensitivity to Light | 70 | 11 | 0.011 |
Bilirubin | 66 | 26 | 0.042 |
Table 1: Symptoms that occur with enhanced frequency in the anemia data set, compared with the control set, which are also known to be highly common in preeclampsia.
So all these analyses lead me to a bold generalization as follows: both preeclampsia and extreme adverse reactions to vaccines are a consequence of a profuse over-production of nitric oxide by eNOS in the artery wall. This excess nitric oxide reacts with cobalamin, which helps to neutralize its effects, but also gets taken down in the process. The blood enters a severe state of crisis following the sudden depletion of cobalamin, leading to the symptoms of pernicious anemia. eNOS is prevented from producing sulfate, and it may well be the sulfate depletion that is at least as big a problem as the overproduction of nitric oxide.
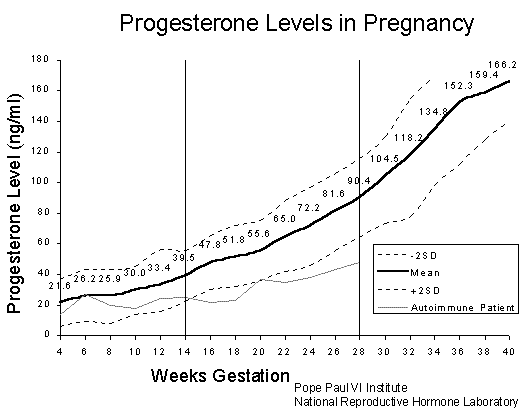
But, if you’re paying attention, you should be asking the question at this point, “How does preeclampsia end up with exuberant synthesis of nitric oxide?” I was puzzled, too, by this question, but the parallels in the symptoms make me think it must be true. I suspect it has something to do with progesterone. As you can see from the figure [25], progesterone levels shoot way up in the last trimester of pregnancy. Progesterone has a remarkable ability to prevent cells from storing cholesterol in private stores [6]. Excess progesterone in the blood would therefore cause the endothelial cells lining the artery wall to give up their cholesterol for the greater good. As we’ve seen from my previous blog post, insufficient membrane cholesterol leads to runaway leaks of small ions, and a rapid influx of calcium, which stimulates eNOS to switch from synthesizing sulfate to synthesizing nitrate. This idea has strong support from the literature [17, 22], where it has been shown that excess nitrates in the blood are associated with preeclampsia, and the amount of nitrate correlates with the severity of the disease.
| Reaction | Count Anemia | Count Control | P-value |
Sleep Disorder | 534 | 140 | 0.0001 |
Seizure | 1144 | 632 | 0.0005 |
Nerve Injury | 69 | 0 | 0.004 |
Disorientation | 112 | 32 | 0.01 |
Chest Pain | 1366 | 278 | 2.0E-7 |
Heart Rate Irregular | 963 | 279 | 1.0E-5 |
Heart Failure | 64 | 8 | 0.01 |
Myalgia | 981 | 416 | 0.00001 |
Paralysis | 384 | 71 | 0.0001 |
Dysphagia | 353 | 96 | 0.0005 |
Loss of Consciousness | 832 | 447 | 0.001 |
Death | 180 | 74 | 0.01 |
Table 2: Other symptoms that were identified as highly signficantly over-represented in the anemia data set, besides those specifically associated with autism or preeclampsia.
The whole point of it might simply be to spare the consumption of cholesterol and sulfur by these cells so that the fetus can have more. Such problems would only arise when there isn’t enough cholesterol sulfate to go around. Interestingly, Triton X-100, a surfactant ingredient found in the flu vaccine, has the same property as progesterone in interfering with cholesterol homeostasis in cells.
A Role for SeizuresYou might imagine that preeclampsia is a precursor to eclampsia, and, if so, you would be right. You may have also noticed that “seizures” was one of the conditions that showed up with a highly significant bias (p= 0.00047) in the Anemia data set compared to the control. In rare cases, preeclampsia turns into eclampsia, a condition that is defined by the appearance of seizures. This to me is extremely intriguing, because it leads me to hypothesize that the purpose of the seizures is to generate sulfate anions. I believe this because I believe that sulfate deficiency is the root cause of preeclampsia.
How might a seizure produce a sulfate anion, and where might it come from? I think the answer is taurine! Taurine is a very unusal amino acid, the only sulfonated amino acid, and it is believed to be basically inert – an end product that happens to be stored in high concentrations throughout life in the heart, brain, and liver. These are arguably the three most important organs for survival in an emergency. I can’t imagine that these organs maintain an abundant store of a molecule that they find useless except possibly for reacting to osmosis imbalances by moving it around between cells and the blood stream. I believe instead that taurine is brought into play under conditions of extreme stress; in particular, under conditions where severe sulfate deficiency would result in a melt-down of the blood if not immediately corrected.
The sulfur in taurine is in a +5 oxidative state. It needs to be in a +6 oxidative state in order to happily live in a sulfate anion. That is, it needs to give up an electron. One thing that an electric current is very good at is pursuading molecules to give up electrons. A seizure induces an electric current! Oxygen molecules (O2) will be happy to pick up electrons given up by other molecules, thus turning into superoxide, a highly reactive molecule. I’m imagining that, in the context of an electric current, a superoxide anion steals a sulfur along with two oxygen molecules from taurine, leaving behind acetaldehyde in its wake. The sulfur atom, now at a +6 charge, is very happy to double-bond with the superoxide, magically yielding a sulfate anion! And this helps solve the brain’s crisis involving insufficient sulfate buffering. So, if this argument is right, there is a silver lining in seizures, in that they can replenish sulfate in the brain.
SummaryThis blog post turned out to be a lot longer than I was expecting when I set out to write it. I’ve hit upon a number of topics, all closely intertwined in somewhat subtle ways. I started by showing the remarkable parallels between preeclampsia and cardiovascular disease, and I argued that the activities going on in cardiovascular plaque are the same as those going on in the arteries of the placenta in preeclampsia, and that both serve the purpose of producing cholesterol sulfate for their host: the heart in the one case and the fetus in the other.
My second topic was the relationship between pernicious anemia and severe adverse reactions to vaccines. Pernicious anemia is a direct result of extreme cobalamin deficiency, and in this section I showed how cobalimin would likely be destroyed if there was an overabundance of nitric oxide in the blood. This overabundance would be due to the stimulation of eNOS to produce nitric oxide in the context of both endotoxin and aluminum in the vaccine. In the case of preeclampsia, the trigger to synthesize abundant nitric oxide is brought on by a depletion of membrane cholesterol in the endothelial cells lining the artery wall. This in turn is caused by progesterone, which is well-known as an agent that leaches choesterol from cell walls, and which rises to high serum levels towards the end of pregnancy.
In the next section, I developed an argument based on studies of the VAERS database, where I showed remarkable links among autism, extreme adverse reactions to vaccines, preeclampsia, and pernicious anemia. I believe that all four of these conditions are attributable to a deficiency in cholesterol sulfate, which results in a dramatic switch from sulfate to nitrate as the anion of choice to maintain electrolyte balance in the blood. An instability brought on by the rapid depletion of cobalamin leads to the symptoms of pernicious anemia. But I think the severe depletion of sulfate in the blood may cause the disturbing symptoms that show up in extreme cases, such as paralysis, seizures, and death.
The final section addressed specifically the topic of seizures, and proposed a novel theory for a positive role seizures may play in allowing taurine to free up its sulfonate and convert it into sulfate. This is of course a critical step in recovery from the event, and so, if I’m right about this speculation, seizures likely are an important part of the solution in such situations.
References[1] U. Acharya, J.-T. Gau, W. Horvath, P. Ventura, C.-T. Hsueh and W. Carlsen, “Hemolysis and hyperhomocysteinemia caused by cobalamin deficiency: three case reports and review of the literature,” J Hematology and Oncology 1:26, Dec. 18, 2008.
[2] A.D. Attie, J.P. Kastelein and M.R. Hayden, “Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis,” J. Lipid Res 42:1717-1726, 2001.
[3] M Brouwer, W Chamulitrat, G Ferruzzi, DL Sauls and JB Weinberg, “Nitric oxide interactions with cobalamins: biochemical and functional consequences” Blood 88:1857-1864, 1996.
[4] A. Cauwels, B. Janssen, E. Buys, P. Sips, and P. Brouckaert, “Anaphylactic shock depends on PI3K and eNOS-derived NO,” J. Clin. Invest. 116:2244-2251, 2006.
[5] L.C. Chappell, P.T. Seed, A.L. Briley, F.J Kelly, et al., “Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial,” The Lancet 354:810-816, Sep 4, 1999.
[6] P. Debry, E.A. Nash, D.W. Neklason and J.E. Metherall, “Role of Multidrug Resistance P-glycoproteins in Cholesterol Esterification,” J. Biol Chem 272(2):1026-1031, Jan 10, 1997.
[7] J.S. Gilbert, M.J. Ryan, B.B. LaMarca, M. Sedeek, S.R. Murphy and J.P. Granger, “Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction,” Am J Physiol Heart Circ Physiol 294:H541-H550, 2008.
[8] H. Kondo, M.L. Osborne, J.F. Kolhouse, M.J. Binder, et al., “Nitrous Oxide Has Multiple Deleterious Effects on Cobalamin Metabolism and Causes Decreases in Activities of Both Mammalian Cobalamin-dependent Enzymes in Rats,” J.Clin.Invest. 67:1270-1283, May 1981.
[9] P.T. Kovanen, “Atheroma formation: defective control in the intimal round-trip of cholesterol,” Eur Heart J 11 (suppl E): 238-246, 1990.
[10] Helen Kay, MD, D. Michael Nelson, MD, Yuping Wang, MD., Ed., The Placenta: From Development to Disease, Blackwell Publishing, Ltd., 2011.
[11] B. Lin, K. Kubushiro, Y. Akiba, Y. Cui, K. Tsukazaki, S. Nozawa and M. Iwamori, “Alteration of acidic lipids in human sera during the course of pregnancy: characteristic increase in the concentration of cholesterol sulfate,” Journal of Chromatography B, 704, 99-104, 1997.
[12] C.J. Lowenstein and T. Michel, “What’s in a name? eNOS and anaphylactic shock” J Clin Invest. 116(8): 2075-2078 Aug 1, 2006.
[13] G. Makedos, A. Papanicolaou, A. Hitoglou, I. Kalogiannidis, A. Makedos, V. Vrazioti, and M. Goutzioulis, “Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia,” Arch Gynecol Obstet (2007) 275:121-124.
[14] J.R. Mann, S. McDermott, H. Bao, J. Hardin and A.Gregg, “Pre-eclampsia, birth weight, and autism spectrum disorders.” J Autism Dev Disord. 40(5):548-54, May 2010.
[15] K.S. McCully, “Chemical Pathology of Homocysteine. V. Thioretinamide, Thioretinaco, and Cystathionine Synthase Function in Degenerative Diseases,” Annals of Clinical and Laboratory Science, 41:4, 300-313, 2011.
[16] R. Mukherjee and N.E. Brasch, “Kinetic Studies on the Reaction between Cob(I)alamin and peroxynitrite: Rapid Oxidation of Cob(I)alamin to Cob(II)alamin by Peroxynitrous Acid,” Chem. Eur. J. 17:11723-11727, 2011.
[17] N. Pathak, H. Sawhney, K. Vasishta and S. Majumdar, “Estimation of Oxidative Products of Nitric Oxide (nitrates, nitrites) in Preeclampsia,” Australian and New Zealand Journal of Obstetrics and Gynaecology, 39(4):484-487, Novr 1999.
[18] C.J.I. Raats, J. Van Den Born, and J.H.M. Berden, “Glomerular heparan sulfate alterations: Mechanisms and relevance for proteinuria,” Kidney Int 57:385-400, 2000.
[19] W.V. Rodrigueza, J.J. Wheeler, S.K. Klimuk, N. Kitson and M.J. Hope, “Transbilayer Movement and Net Flux of Cholesterol and Cholesterol Sulfate between Liposomal Membranes,” Biochemistry 34, 6208-6217, 1995.
[20] N. Sattar and I.A. Greer, “Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening?” BMJ 325(7356):157-160, 2002.
[21] N. Sattar, A. Bendomir, C. Berry, J. Shepherd, I.A. Greer, and C.J. Packard, “Lipoprotein subfraction concentrations in preeclampsia: Pathogenic parallels to atherosclerosis,” Obstetrics and Gynecology 89:3 403-408, Mar 1997.
[22] A.Smarason Jr., K.G. Allman, D. Young and C.W.G. Redman, “Elevated levels of serum nitrate, a stable end product of nitric oxide, in women with pre-eclampsia,” BJOG 104(5):538-543, May 1997.
[23] R.S. Sprague, A.H. Stephenson, E.A. Bowles, M.D. Stumpf and A.J. Lonigro, “Reduced Expression of Gi in Erythrocytes of Humans With Type 2 Diabetes Is Associated With Impairment of Both cAMP Generation and ATP Release,” Diabetes 55:12, 3588-3593 Dec. 2006.
[24] H. Yanai, N.B. Javit, Y. Higashi, F. Hirotoshi and C.A. Strott, “Expression of Cholesterol Sulfotransferase (SULT2B1b) in Human Platelets,” Circulation 109:92-96, 2004.
[25] From “Progesterone Levels during Pregnancy,” Center for Reproductive Immunology and Genetics; http://repro-med.net/repro-med-site2/index.php?option=com content&view=article&id=25&Itemid=12
